Osteogenesis Imperfecta (OI) is a rare hereditary bone disease (Figure 1). Children with OI can easily cause severe bone fracture even with a slight collision, and therefore called "porcelain dolls." Currently there are no effective drugs for OI treatment. Drugs for these rare diseases are defined as "orphan drugs" by the U.S. FDA. The Chinese drug regulatory agencies also include OI in the list of rare diseases. Successful designation for orphan drug status by the U.S. FDA will bring a series of benefits for the follow-up drug development, including reduction for the number of samples in clinical trials, opportunity for speeding up the review process, fee reduction or waive for marketing authorization, and 7 years market exclusivity for the approved product.
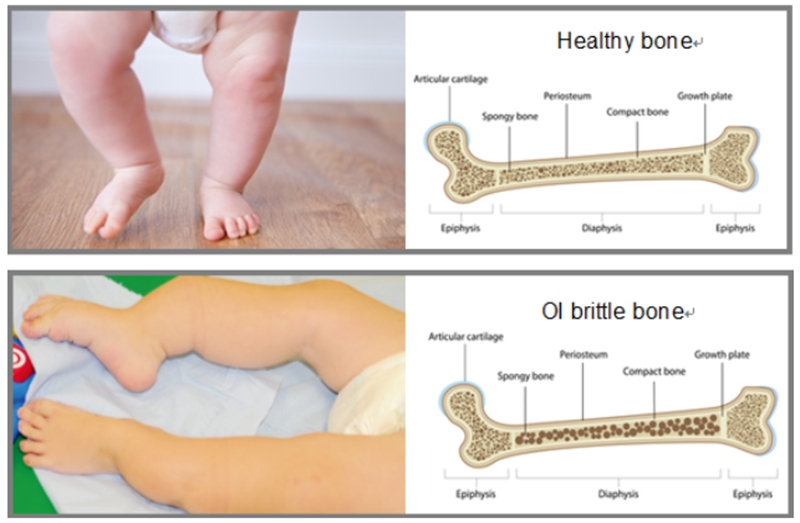
Figure 1 The differences of bone structure between healthy people and patients with osteogenesis imperfecta.
Notes:Figure 1 was adopted from Joan C. Marini, et al. Osteogenesis imperfecta. Nature Reviews 3: 17052-17072 and <Do not correct your baby's frog legs>.
A novel aptamer drug was granted for orphan drug designation (ODD) by the U.S. FDA on August 19, 2019 for the treatment of OI ( DRU-2019-6966). This novel aptamer drug was jointly discovered by the research group of Professor Ge Zhang from Law Sau Fai Institute for Advancing Translational Medicine in Bone & Joint Disease, Hong Kong Baptist University (TMBJ: http://tmbj.hkbu.edu.hk/) and the research group of Professor Aiping Lyu from the Institute of Integrated Bioinformedicine and Translational Science, Hong Kong Baptist University (IBTS: http://ibts.hkbu.edu.hk/). This project was supported by the University-Industry Collaboration Programme of the Innovation and Technology Commission, Hong Kong SAR Government.
Prof. Ge Zhang is the leader of this project. He is the deputy director of Law Sau Fai Institute for Advancing Translational Medicine in Bone & Joint Disease, Hong Kong Baptist University (TMBJ: http://tmbj.hkbu.edu.hk/). “Genetic evidences demonstrate that inhibition of sclerostin could improve the phenotype of OI, but clinical evidence suggests that there is still a significant non-negligible cardiovascular risk in inhibiting sclerostin by monoclonal antibody. Our mechanism studies found that sclerostin participates in the inhibition of bone formation and protection of cardiovascular system through different domains. Therefore, we strategically screened and optimized a new class of molecule-nucleic acid aptamer against sclerostin protein. This nucleic acid aptamer can be expected to, not only significantly promotes bone formation in OI mice, and does not affect the cardiovascular protection of sclerostin and so does not increase cardiovascular risk." Prof. Zhang said.
Dr. Kennedy Y.H. Wong Endowed Chair Professor, Prof. Aiping Lyu is the co-leader of this project. He is the director of the Institute of Integrated Bioinformedicine and Translational Science, Hong Kong Baptist University (IBTS:http://ibts.hkbu.edu.hk/). “Aptamer screening is the process of identifying the most optimal molecule from a random single strand DNA library with more than 1015 sequences, just like looking for a needle in the haystack. In this project, we applied a high-throughput screening methodology by employing the artificial intelligence (AI) technology in the screening of aptamers against sclerostin. This technology can reduce the manpower, shorten the screening time and reduce the reagent consumption. More importantly, systematic analysis to the candidate sequences using AI technology can help to identify the optimal candidates quickly and efficiently, avoiding the loss of potential promising candidates which happens commonly in traditional analytical methods.” Prof. Lyu introduced.
Dr. Yuanyuan Yu, director of Cellular & Molecular Biology Laboratory of Guangdong-Hong Kong- Macao Greater Bay Area International Research Platform for Aptamer-based Translational Medicine and Drug Discovery (HKAP, https://www.hkaptamer.com/) is one of the investigators in this project. “Aptamers are artificial single stranded oligonucleotides or peptides. Aptamers bind to their targets through three dimensional-structural complementarities with high binding specificity and affinity. The specificity and affinity of aptamers are comparable to therapeutic monoclonal antibodies, but aptamers have some major advantages over monoclonal antibodies, for example, better stability, lower batch-to-batch variations and lower immunogenicity. Aptamers can be selected, amplified and enriched through a process called Systematic Evolution of Ligands by EXponential enrichment (SELEX). The selection process can be tailored-made according to the requirements of diverse targets. For example, we can design positive selection and counter selection to develop aptamer candidates which can specifically bind to the positive targets, while remove the sequences which can bind to the negative targets. In this project, we strategically designed the tailored selection for aptamer selection against sclerostin based on the different roles of sclerostin domains. The identified aptamer candidate not only can improve the phonotype of OI mice, but also not affect the cardiovascular system.” Dr. Yu said.
Investigator Dr. Shuaijian Ni, the director of chemical synthesis laboratory of HKAP (https://www.hkaptamer.com/) mentioned “Aptamers identified by SELEX are composed of natural bases. They can be easily degraded by the nucleus in the circulation and hence have low stability. In addition, as the small molecular weight, aptamers can be removed quickly through renal filtration. Therefore, chemical modifications are required to increase the in vivo stability and pharmacokinetic properties. Solid phase synthesis provides a very convenient way for chemical modifications to aptamers. In this project, we prepared chemically modified aptamers against sclerostin.”
Investigator Prof. Kangning Ren, director of microfluidics laboratory and deputy director of HKAP(https://www.hkaptamer.com/)said: “In this project, the microfluidic system designed by our platform was applied in the aptamer screening against sclerostin. Microfluidic is a technique which can precisely control and manipulate fluids in the range of the microliters (especially in the submicro structures). The application of microfluidics to the aptamer selection can increase the screening efficiency exponentially. The reproducibility and success rate of screening can also be significantly improved.”
Prof. Zhenlin Zhang who is the director of the China Society of Osteoporosis and Bone Mineral Research,
director of the Osteoporosis Division of the Sixth People's Hospital Affiliated to Shanghai Jiaotong University, , the author of the famous clinical practice of bone metabolism and bone disease in China give a high comment to this work. "The China Society of Osteoporosis and Bone Mineral Research has issued a guideline for clinical diagnosis and treatment of osteogenesis imperfecta in China in 2019. The new aptamer drug discovered by Prof. Ge Zhang and Prof. Aiping Lyu from Hong Kong Baptist University has granted for orphan drug designation by the US FDA. This has a great significance. It can facilitate the orphan drug development for OI treatment in China and will further promote Shanghai and Hong Kong to jointly develop China's own innovative drugs for OI treatment.”
Prof. Lin Chen is the principle investigator of the National Key R&D Program of China "Study on the influence and mechanism of hereditary calcium and phosphorus metabolic disorders on the development of children's bones and other organs", basic and translational research group leader of China Society of Osteoporosis and Bone Mineral Research, and professor of the Third Military Medical University. "Basic research on rare diseases including bone mineral metabolism, and the translation of new drugs have attracted increasing attention from the Ministry of Science and Technology. In 2018, National Key R&D Program of China has supported RMB 18.46 million to the key project of Development Programming and Metabolic Regulation. As a part of the national innovation system, Prof. Zhang from Hong Kong Baptist University was invited to join the research project. He will apply aptamer technology to both the basic and translational research on the children's genetic calcium and phosphorus metabolism disorders."

Team Members of this Project: (from left to right)
Prof. Zhang Ge (Professor & Deputy Director HKAP)
Dr. Ren Kangning (Assistant Professor; Director of Microfluidics Laboratory & Deputy Director of HKAP)
Dr. Ni Shuaijian (Director of Chemical Synthesis Laboratory, HKAP)
Dr. Yu Yuanyuan (Research Assistant Professor & Director of Cellular & Molecular Biology Laboratory, HKAP)
Dr. Liu Jin (Research Assistant Professor, HKAP)
Mr. Chan Chi Leung (Senior Technical Officer, HKAP )
Ms. Wang Luyao (PhD Candidate, HKAP)
 Aptamer Selection & Characterization
Aptamer Selection & Characterization
 Aptamer Synthesis & Modification
Aptamer Synthesis & Modification

Aptamer Purification

Aptamer Analysis & Quality Control